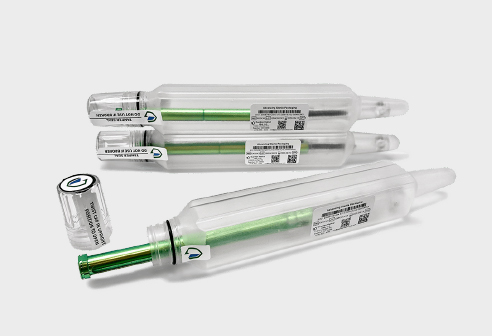
The future of sterile implant
packaging is here, pre-validated
with a 10-year shelf life.

Why CapSure™?
It’s minimal footprint reduces costs and improves efficiencies throughout every segment of the value chain. It’s pre-validated seal eliminates the need for complicated and costly validations, ensuring a faster, more reliable packaging process reducing overall risk and improving useability. CapSure™ sterile barrier packaging systems results in shorter lead-times to commercialization and significant reduction of your carbon footprint throughout the value chain.
Exceptional Protection
CapSure™ is built for unmatched impact resistance and clarity, protecting implants during transport and storage.
Efficiency in the O.R.
Intuitive design and clear packaging enable quick identification and aseptic transfer, crucial for maintaining surgical efficiency.
Sustainable and Economical
With a small footprint and recyclable materials, CapSure™ reduces sterilization, shipping, and storage costs, all while supporting sustainability efforts.

Simplifying Sterile Packaging
Guardian Medical’s packaging solutions go beyond standard offerings. We focus on ease of use, reliability, and efficiency to deliver superior performance in the O.R. Our unique designs simplify the packaging process, reduce lead times, and eliminate the complexities of traditional packaging methods.
No Sealing Equipment Needed
CapSure’s pre-validated packaging means no investment in sealing equipment, reducing overhead and maintenance costs.
CapSure’s packaging eliminates the need for in-process seal integrity testing equipment, improving operational efficiencies and robust in-process QC monitoring.
Short Lead Times
CapSure’s pre-validation data reports are available to reduce your lead-time to commercial launch. Our off-the-shelf packaging kits are readily available ensuring improved delivery and less risk to your supply chain
Simplifying
Sterile Packaging
Guardian Medical’s packaging solutions go beyond standard offerings. We focus on ease of use, reliability, and efficiency to deliver superior performance in the O.R. Our unique designs simplify the packaging process, reduce lead times, and eliminate the complexities of traditional packaging methods.
No Sealing Equipment Needed
CapSure’s pre-validated packaging means no investment in sealing equipment, reducing overhead and maintenance costs.
CapSure’s packaging eliminates the need for in-process seal integrity testing equipment, improving operational efficiencies and robust in-process QC monitoring.
Short Lead Times
CapSure’s pre-validation data reports are available to reduce your lead-time to commercial launch. Our off-the-shelf packaging kits are readily available ensuring improved delivery and less risk to your supply chain
Stay Informed
Stay Informed
We focus on ease of use, reliability, and efficiency to deliver superior performance in the O.R. Our unique designs simplify the packaging process, reduce lead times, and eliminate the complexities of traditional packaging methods

Sterile Packaging as a Growth Strategy: Turning Cost Centers Into Strategic Advantages

Sterile Packaging that Supports Nurses in the OR

From the Supply Chain to the Operating Room: How Footprint Reduction Impacts Every Step
Meet Us at
Our Upcoming Events
Join our team at upcoming events to explore our solutions and discover how we can support your packaging needs.
Join Our Newsletter
Subscribe to the Guardian Medical newsletter and never miss an update. Get the latest updates on our packaging solutions, industry insights, and event invitations. Stay connected and stay informed.