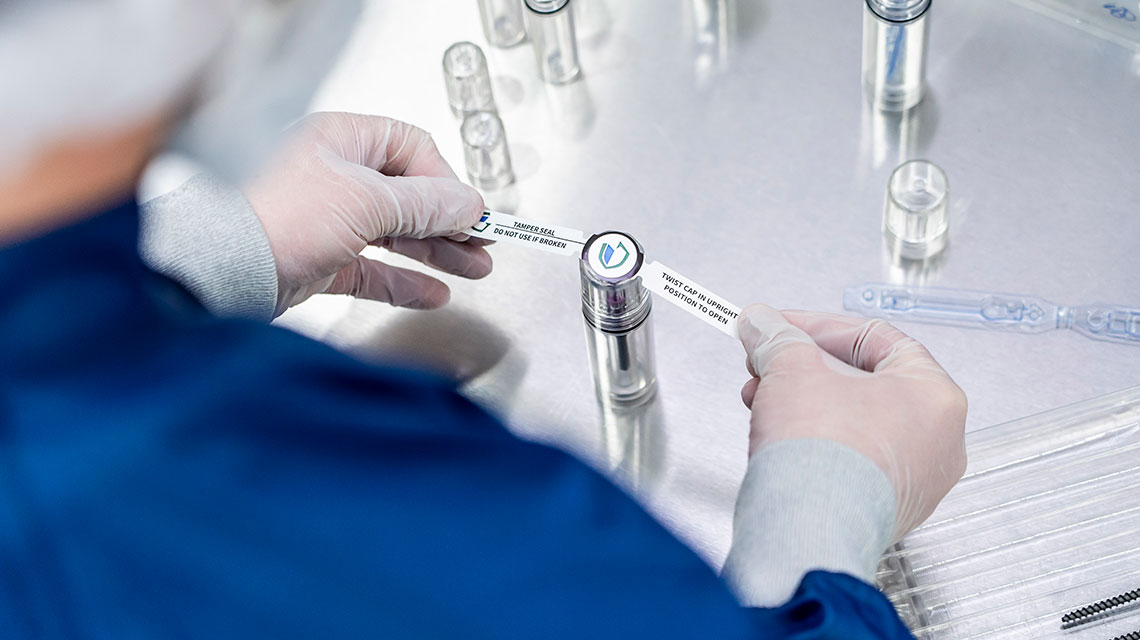
Sterile packaging has traditionally been viewed as a necessary burden: an unavoidable expense, a validation hurdle, a space constraint, and too often a source of costly delays. But that perception is changing. OEMs navigating supply chain constraints, rising costs, and pressure for faster market launches are discovering that sterile packaging has untapped strategic power.
Ready to Simplify
your sterile packaging?
Contact us today to see how we can make a difference in your operations.