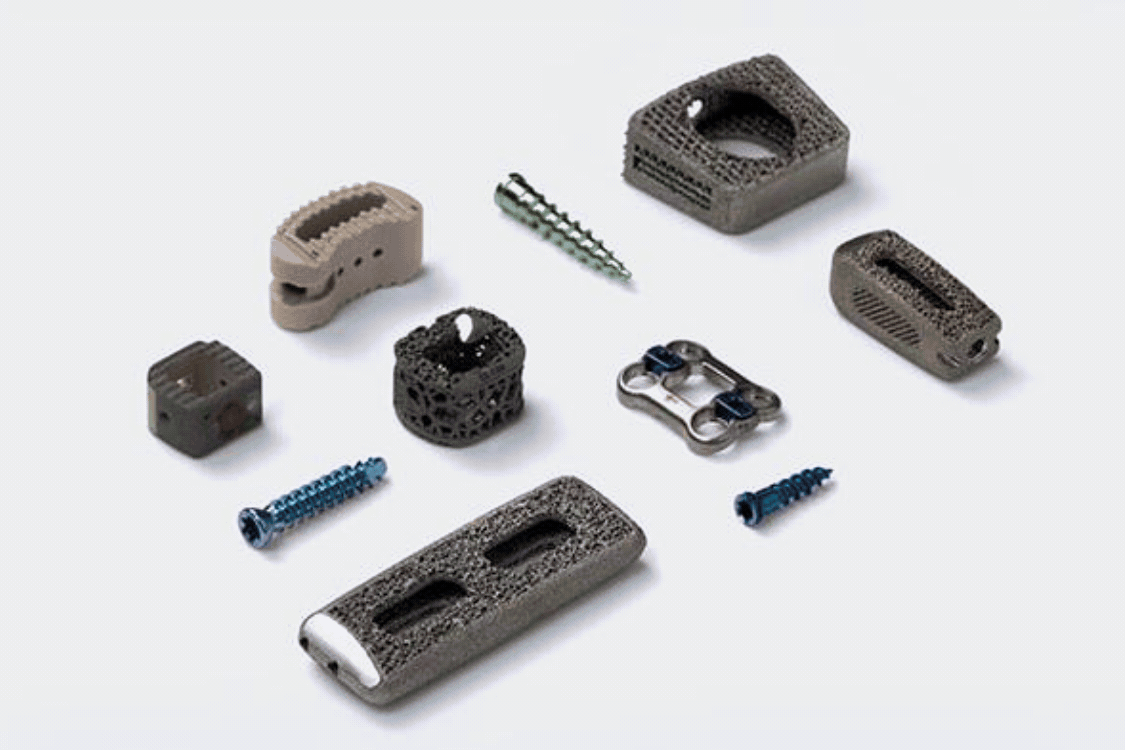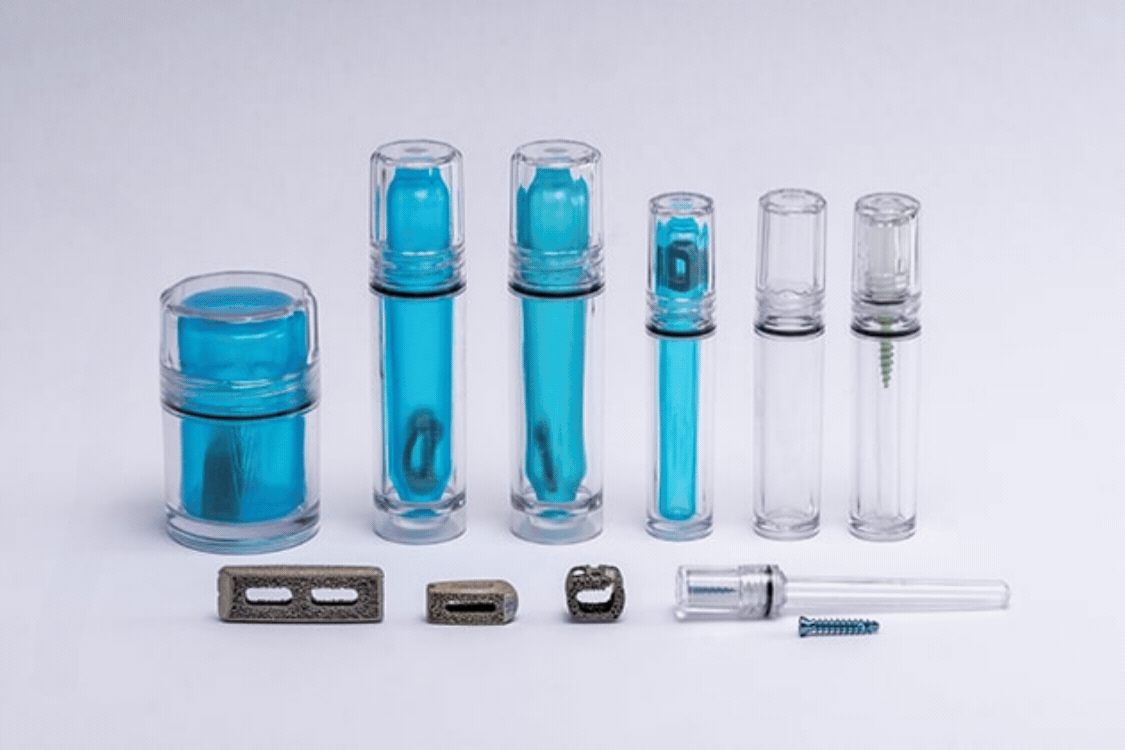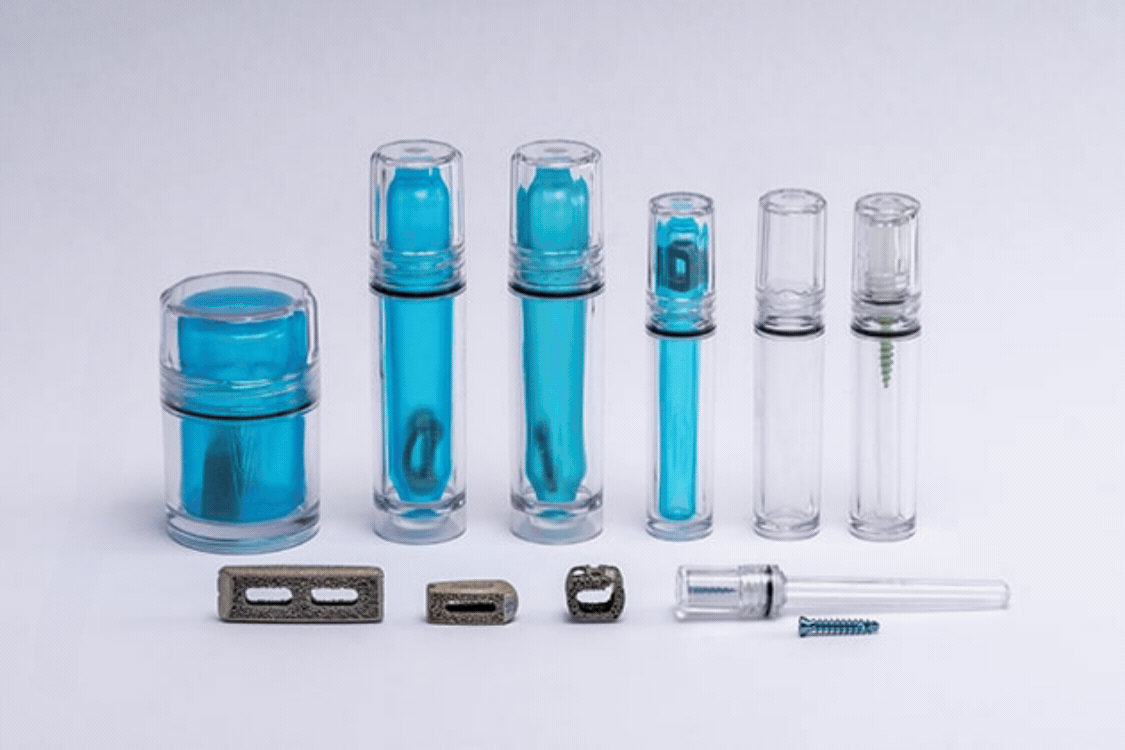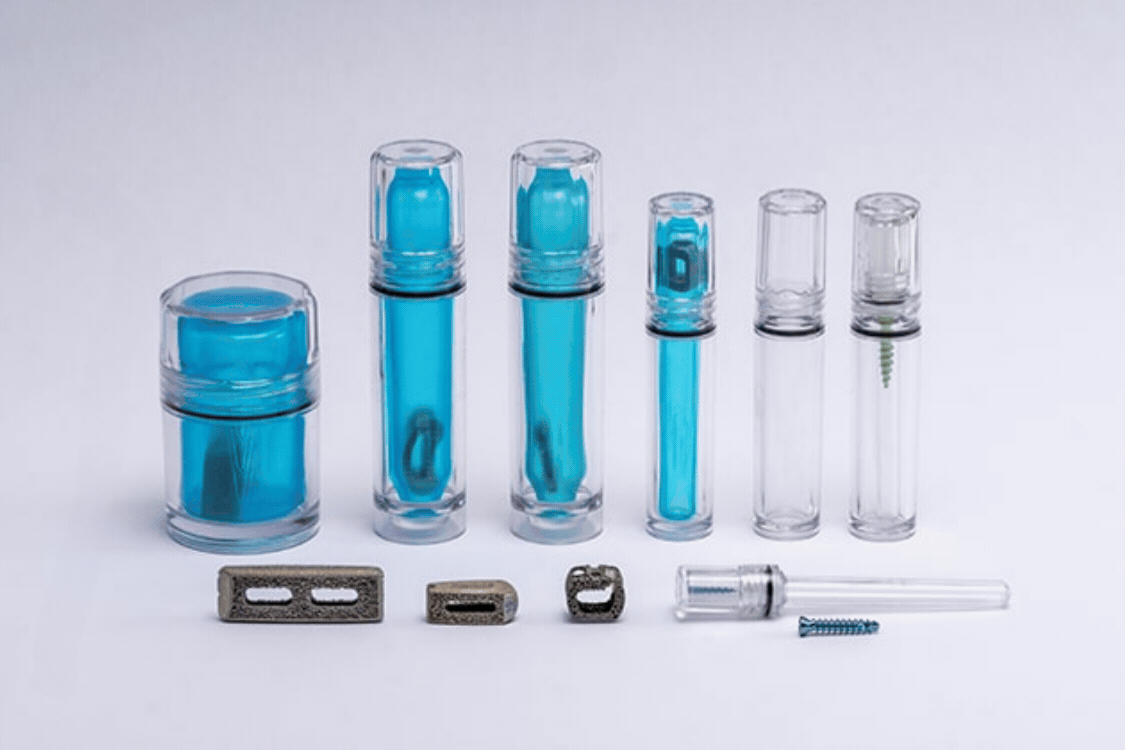
Spinal implant devices require the highest level of risk mitigation, product safety, and sterility assurance to protect patient health. Guardian Medical's CapSure™ sterile barrier packaging tubes and jars are optimizing the entire value chain for interbody fusion cages. From improving manufacturing efficiencies to reducing sterilization and shipping costs, CapSure™ provides a robust, protective package that minimizes footprint and is intuitive to use, resulting in a user-friendly implant packaging system.
Challenges in Traditional Implant Packaging
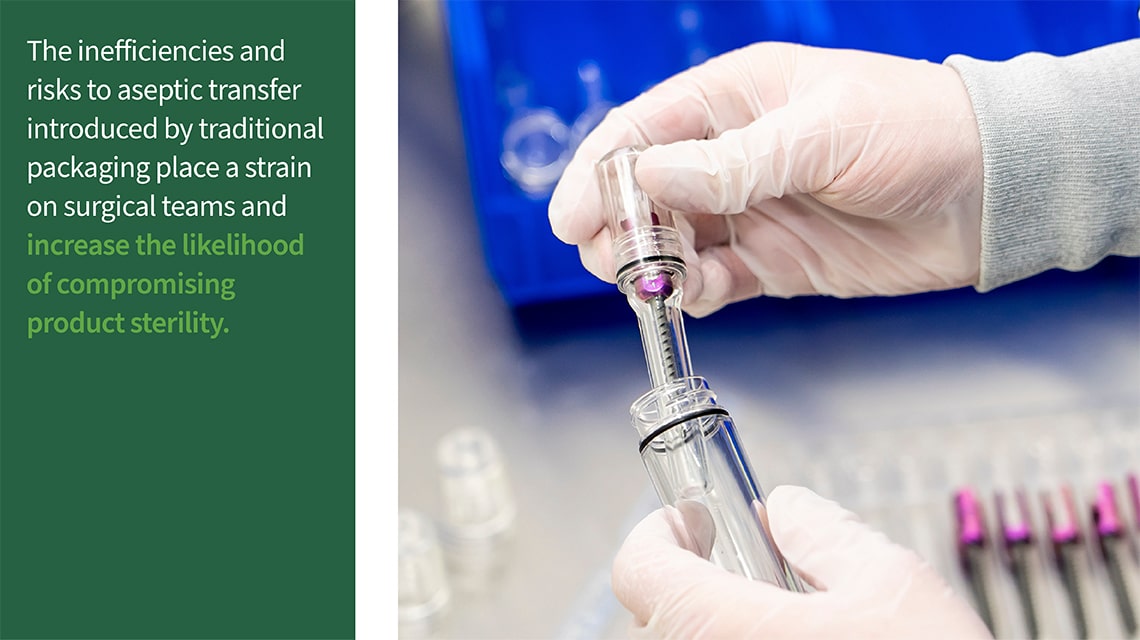
Traditional spinal implant packaging systems have long been used to protect sterile implants throughout distribution until their final use. However, these systems still experience performance failures and are often complex and difficult to open, making consistent and reliable aseptic transfer techniques challenging and posing a risk to patient safety.
The packaging footprint for spinal implants and interbody fusion cages plays a crucial role in manufacturing efficiency, sterilization and logistics costs, and inventory management. Current implant packaging systems are bulky and not optimized for the entire value chain, leading to challenges in the operating room. The larger footprint of traditional packaging systems results in higher costs for sterilization, logistics, and inventory management
Guardian Medical's CapSure™ pre-validated sterile packaging systems significantly reduce packaging footprint and require no capital equipment for package sealing. They utilize a simple go/no-go fixture for in-process seal integrity verification, which provides a significant time and cost advantage. This innovation improves efficiency and quality assurance and eliminates the need for additional in-process seal integrity testing, such as bubble leak or dye penetration testing.
The Impact on the Value Chain
These challenges are not just logistical—they directly impact patient outcomes at the point of use. When sterile barriers are compromised, it can result in infections, extended hospital stays, and even the need for additional surgeries. The inefficiencies and risks to aseptic transfer introduced by traditional packaging place a strain on surgical teams and increase the likelihood of compromising product sterility.
At Guardian Medical, we understand these challenges and have developed the CapSure™ packaging system to address every aspect of the value chain. CapSure™ is designed to reduce the size and weight of packaging while simplifying the packaging process, offering a pre-validated, minimal footprint solution that enhances the safety and efficiency of spinal implants. It provides an intuitive, easy-to-use package that surgeons appreciate.
Our commitment to innovation and patient safety is at the core of what we do. CapSure™ not only protects the integrity of medical implants but also streamlines the value chain, contributing to better outcomes for manufacturers, distributors, surgeons, and patients.
To learn more about how Guardian Medical’s CapSure™ packaging can revolutionize your spinal surgeries, contact us today. Together, we can overcome the challenges of traditional packaging and ensure the best possible outcomes for your products and our patients.