Sterile packaging in orthopedic device manufacturing, supply chain, and surgeries is rapidly evolving, with increasing demands for efficiency, safety, and traceability. Recent innovations in tamper-evident designs, UDI (Unique Device Identification) traceability, and inventory management are shaping the future of how medical devices are stored, tracked, and deployed in surgical settings.
Tamper-Evident Packaging: Ensuring Safety and Trust
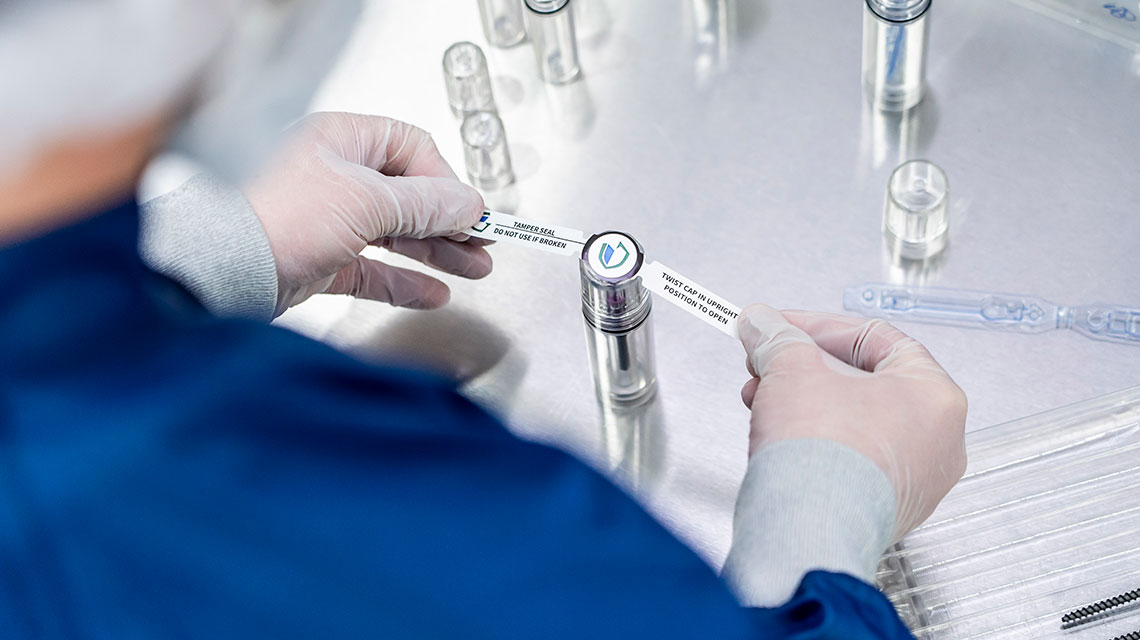
In the realm of sterile packaging, tamper evidence is more than a convenience, it's a necessity. Surgical teams need confidence that medical devices have not been compromised before use. Tamper-evident features in packaging provide a clear indication the sterile barrier has not been breached. This is particularly crucial in orthopedic surgeries, where implants and surgical tools must be sterile and intact to avoid contamination or infection.
Tamper-evident packaging designs now include robust visual indicators, such as color-coded seals and materials that show obvious signs of tampering. These solutions increase efficiency in the OR by allowing the surgical team to verify the sterility and safety of devices quickly. Guardian Medical's CapSure sterile barrier packaging system offers an efficient and effective solution for tamper proofing devices.
UDI Traceability: A Game-Changer for Device Management
The FDA’s UDI rule mandates that every medical device must have a unique identifier that can be tracked throughout its lifecycle. For orthopedic surgeries, where a vast array of implants are involved, UDI traceability has become critical for inventory control and ensuring the right device is used for each patient.
UDI labels are now integrated into sterile packaging, allowing for seamless scanning and tracking from the manufacturer to the operating room. This traceability ensures that hospitals maintain compliance with regulatory standards while also streamlining recall processes if necessary. A well-managed UDI system ensures that surgical staff can easily locate specific implants, reducing errors and increasing the efficiency of inventory management. Guardian Medical can manage UDI labeling for brand owners through their robust processes and procedures.
Smarter Inventory Management: Reducing Waste and Costs
Inventory management is a growing concern in orthopedic surgeries. With hundreds of implants often needing to be available for a single procedure, OEM's, Reps, Hospitals and or ASC's must efficiently store and manage these devices to avoid waste, reduce costs, and ensure patient safety. Traditionally, bulky sterile packaging has taken up valuable storage space, leading to inefficiencies in inventory management and increased costs.
Guardian Medical's sterile packaging solutions are designed with minimal footprints, allowing more devices to be stored in the same space without compromising protection. Innovations in labeling, such as tamper-evident and color coded packages, also make it easier for staff to quickly identify the right implant making it more efficient to manage inventory and reducing costs. A reduced footprint is saving brand owners significant costs to manage and transport inventory due to the space savings CapSure offers compared to traditional packaging systems.
How Guardian Medical is Incomparable to Traditional Packaging Solutions
At Guardian Medical, we understand the evolving needs of the orthopedic industry, which is why we’ve developed innovative packaging solutions that outperform traditional sterile packaging. Our CapSure™ packaging system leads the way in several critical areas throughout the entire value chain:
- Minimal Footprint Design: Guardian Medical's CapSure system reduces the size of packaging without compromising the sterility or safety of the implants. This allows OEMs to store more devices in less space, cutting down on inventory management costs and saving space in the OR.
- Tamper-Evident Seals: With color-coded tamper labels, CapSure packaging offers easy size identification and verification of the seal in the OR. This empowers surgical staff to confidently select and verify the size and sterility of implants with a quick glance.
- UDI Integration: CapSure packaging incorporates UDI traceability, making it easier than ever to track implants from production to the patient. This level of traceability ensures regulatory compliance while giving OEMs greater control over their device inventories.
- Pre-Validated and 10-Year Shelf Life: With a pre-validated 10-year shelf life, CapSure eliminates many of the risks associated with traditional packaging, such as expired inventory or compromised sterility. Our packaging is durable enough to withstand high impacts during transportation, ensuring that the integrity of the packaging and sterility of the implants is maintained from manufacturing to end user.
Guardian Medical’s focus on innovative and efficient packaging solutions makes us the go-to partner for device manufacturers seeking to improve usability, reduce costs, and offer new, disruptive technologies. Ready to elevate your sterile packaging solutions?
To learn more about how Guardian Medical’s CapSure™ can revolutionize your operations and take your sterile packaging to the next level. contact us today.