Nov 14-16 | Booth #911
Heading to NASS?
Let's Meet!

NASS 2025 Starts In
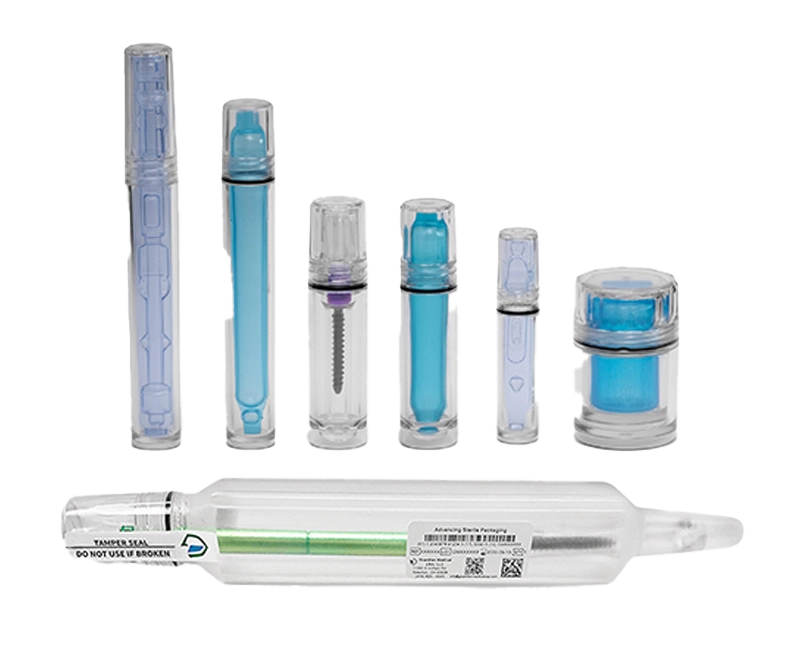
The Challenge
Sterile Packaging Shouldn't Slow Your Launch
Spine implant manufacturers often face delays when selecting sterile packaging. Complex validations, specialized sealing equipment, and long lead-time materials delay commercialization.
At Guardian Medical, our packaging solution is Launch-Ready.
The Solution
Sentinel™ Jars, Smarter Protection for Complex Spine Implants & Valkyrie™ Pedicle Screw Packaging System, The Future of Pedicle Screw Packaging
Our pre-validated Sentinel Jars are designed to fit even the largest ALIF implants without added bulk. Meanwhile, Valkyrie holds both the pedicle screw and corresponding locking screw within a single package, enhancing packaging operational efficiency and reducing the number of packaging components, minimizing waste and cost.
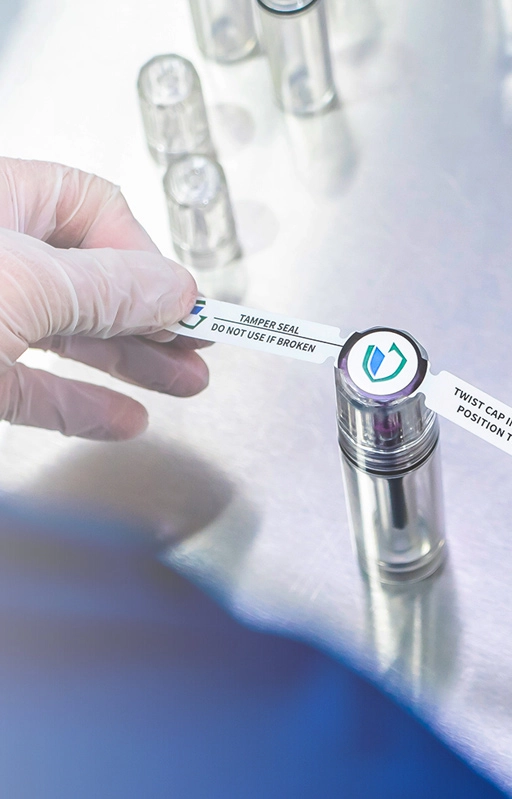
- • Pre-Validated | 10-year shelf life
- • No Specialized Sealing Equipment
- • User-Friendly Aseptic Transfer
- • In-Stock & Ready-to-ship
See Sentinel & Valkyrie firsthand at Booth 911 and experience how we're simplifying sterile packaging for spine OEMs.

Innovation Across the Guardian Medical Portfolio
We’re showcasing the future of sterile barrier packaging, from Sentinel™ Jars for 3D-printed spine implants to Valkyrie™ solutions for pedicle screws and fixation systems.
Each system is purpose-built to reduce footprint, improve handling, and deliver long-term protection, because the OR deserves packaging as advanced as the implant itself.
to Discover our Latest Innovation
Speed, Simplicity, and Confidence, Built In
Guardian Medical’s pre-validated, single-barrier systems are engineered to help you:
Accelerate product launch timelines
Cut packaging, sterilization, and logistics costs
Eliminate sealing equipment and complex validations
Ensure consistent, aseptic presentation in the OR
Support sustainability with recyclable Tritan™ materials



